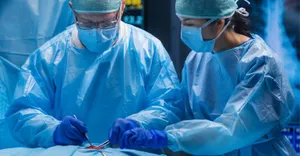
AI-powered language services facilitate better data analysis and insightsAI-powered language services facilitate better data analysis and insights
Precise real-time transcription via speech recognition tech can also free up hospital bed space and reduce patient readmissions.
Subscribe to WHX Insights newsletter
Stay at the forefront of healthcare trends & innovations










.png?width=300&auto=webp&quality=80&disable=upscale)